Question #822dc
1 Answer
The answer is (B)
Explanation:
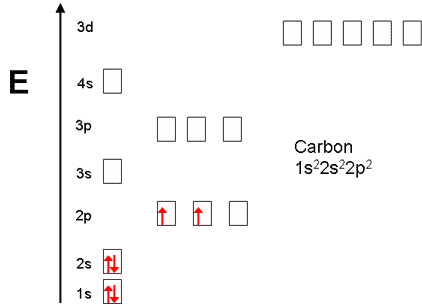
Carbon,
Now, these electrons are distributed on two energy levels
- the first energy level holds two electrons
- the second energy level holds four electrons
Now, the first energy level contains one orbital, the
- one s-orbital, the
#2s# orbital, located in the#2s# subshell- three p-orbitals, the
#2p_x# ,#2p_y# , and#2p_z# orbitals, lcoated in the#2p# subshell
As you know, an orbital can hold a maximum of two electrons. This means that if you distribute carbon's
#1s^2 -># two electrons occupy the#1s# orbital
#2s^2 -># two electrons occupy the#2s# orbital
#2p^2 -># two electrons occupy the#2p_x# and#2p_z# orbitals
Therefore, you can say that carbon has two electrons in its