Question #3ad94
2 Answers
l is a higher energy subshell than k, but they are only theoretical.
Explanation:
These are theoretical subshells because the only ones we've found are s, p, d, and f. This is because each subsequent subshell has more electrons in - s has
We haven't found enough elements in the periodic table to fill up subshells past f, such as g, h, j, k, or l (going alphabetically from f and skipping over i, due to similarities with j).
Maybe one day we'll find these elements with hundreds of electrons so that they fill up all the orbitals down to 7p, and then we'll go to further subshells. But perhaps atoms this large are too unstable to exist.
The L-shell is at a higher energy level.
Explanation:
The electrons in an atom are arranged into "shells" according to their principal quantum number
All electrons with the same value of
The values of
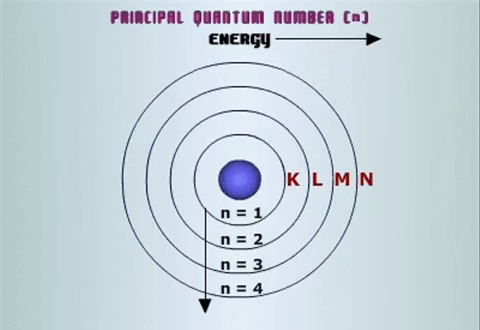
Shells with lower numbers/letters are closer to the nucleus.
Shells that are further out are at a higher energy level, because it takes energy to pull an electron away from the attraction of the nucleus.
The L-Shell is further from the nucleus than the K-shell, so the L-shell is at a higher energy level.


