Question #09e65
1 Answer
I can think of three ways to identify nonequivalent protons.
Explanation:
In general, you identify different sets of equivalent protons. The sets are then groups of nonequivalent protons.
Identify a plane of symmetry in the molecule.
If the protons are interchangeable by refection in a mirror plane, they are equivalent.
Consider cyclohexanol.
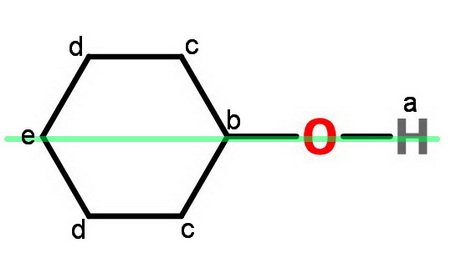
The green line represents a mirror plane perpendicular to the paper.
Proton a, b and e are three nonequivalent sets of 1 proton each.
Protons c are an equivalent set of 4 protons.
So are protons d.
There are 5 sets of nonequivalent protons.
2. Identify an axis of symmetry in the molecule.
If the protons are interchangeable by rotation about an axis, they are equivalent.
Consider methyl chloride.
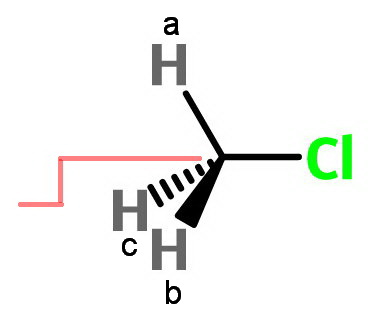
The red crank represents a threefold axis of rotation.
If we rotate 120° clockwise, a→b, b→c, and c→a.
If we rotate 120° counterclockwise, a→c, c→b, and b→a.
Thus a⟷b⟷c, and we have a single set of 3 equivalent protons.
3. Replace each proton in turn by a dummy atom
If replacement of either proton gives the same product, the protons are equivalent.
Consider 1-chloropropane.
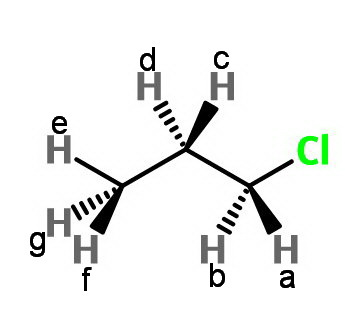
Replacing either
Replacing either
Replacing
There are 3 sets of nonequivalent protons.

