A #"1.32 L"# volume of gas has a pressure of #"1.00 atm"#. What will the volume be if the pressure is increased to #"30.0 atm"#?
1 Answer
Nov 8, 2016
The final volume will be
Explanation:
This question involves Boyle's law, which states that the volume of a given amount of gas varies inversely to its pressure as long as the temperature and mass remain constant.
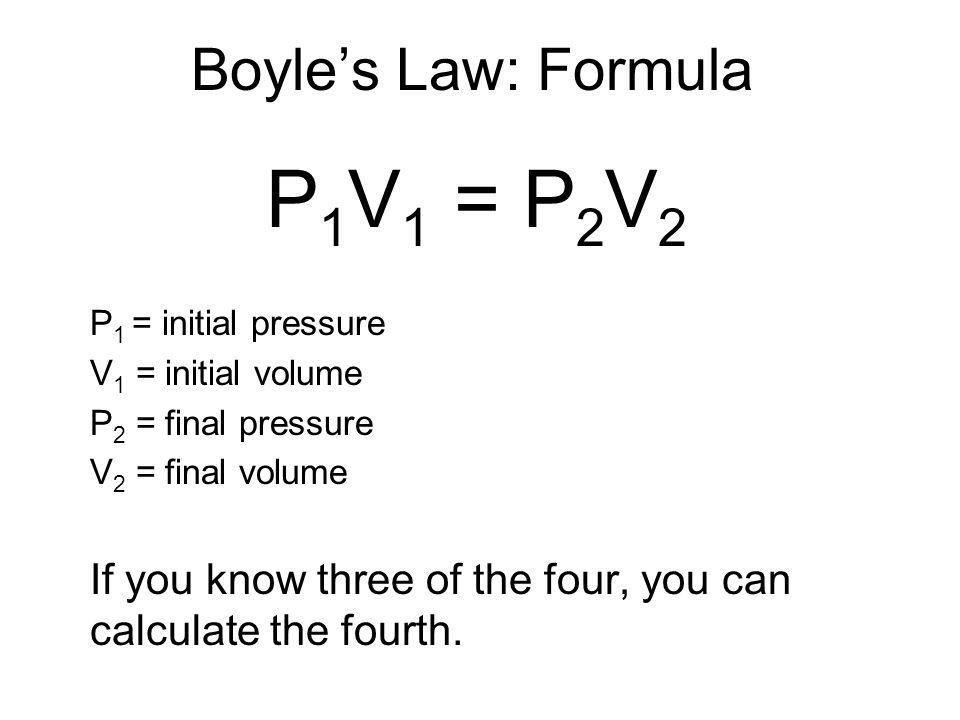
Solution
Rearrange the equation to isolate

