Question #64165
1 Answer
Dec 19, 2016
Explanation:
* I am going to assume that you mean a 11.0 L sample of
To find the final volume of chlorine gas we can use Boyle's Law:
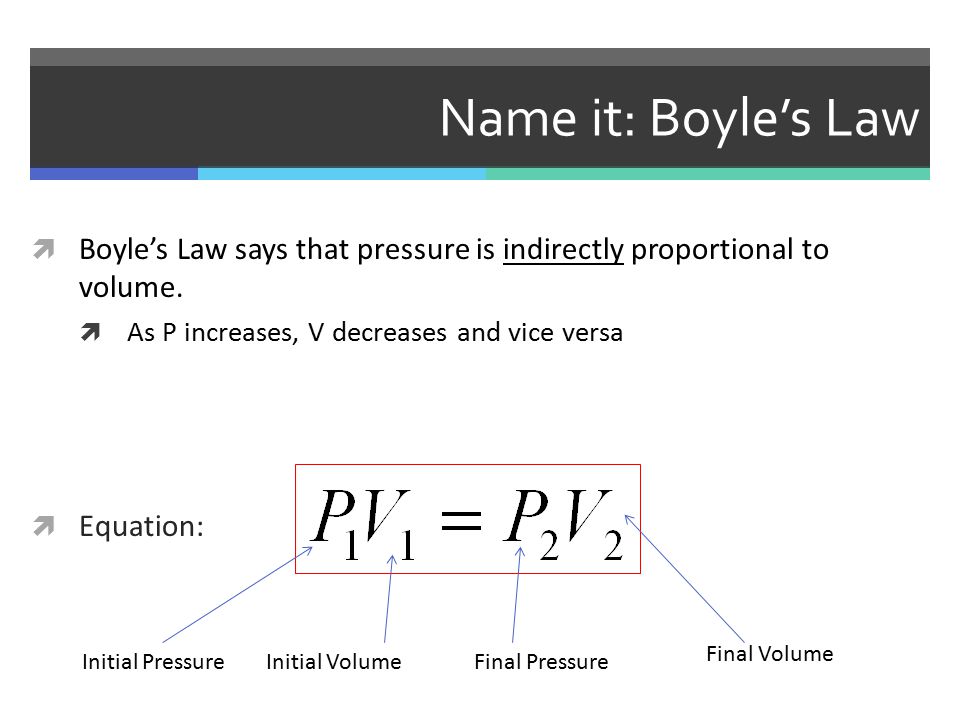 http://slideplayer.com/slide/5281881/
http://slideplayer.com/slide/5281881/
Based on the information you've given me, we know the following variables:
Since we don't know what
Now, just plug the given values into the equation:

