Why are electrons less repulsive when in a bond than when as a lone pair?
1 Answer
Because electrons need to be in a more particular space in a bond than when they are merely in an orbital.
A lone pair of electrons is simply a pair of electrons in a valence orbital that is not being used to bond at the time.
However, electrons in a bond must be in between the two atoms. That way, the repulsive forces of the nuclei of atom A and B are shielded at the equilibrium bond distance:
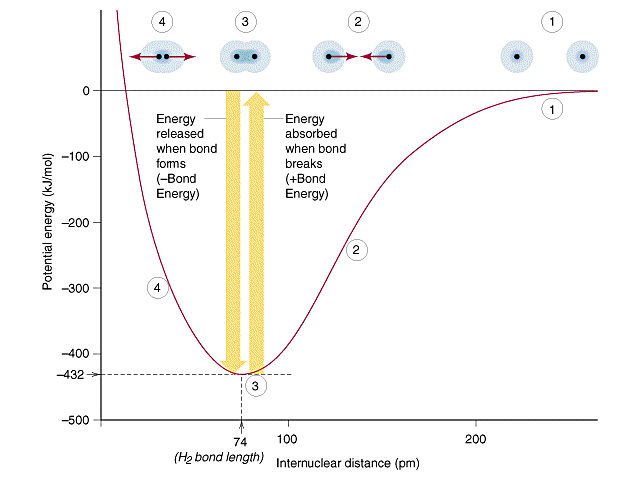
Otherwise, all we have are the nuclear repulsions and electron-nucleus attractions (without the electron shielding), which would put the well in the potential energy curve at a less stable energy at the equilibrium bond distance.
So, they have to be directly in between the atoms as much as possible. Therefore, they are in a more confined space than in a lone pair.

