Question #228f8
1 Answer
WARNING! Long answer! Here's what I get.
Explanation:
1,1-Dichlorocyclohexane (A)
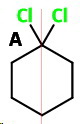 1,1
1,1
This has no chiral carbons, so it is achiral and optically inactive.
cis-1,2-Dichlorocyclohexane (B)
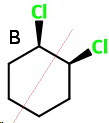 cis-1,2
cis-1,2
This has two chiral carbons, but it has an internal plane of symmetry that bisects the
It is a meso compound, so it is optically inactive.
cis-1,3-Dichlorocyclohexane (C)
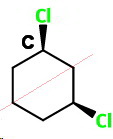 cis-13
cis-13
This has two chiral carbons, but it has an internal plane of symmetry that passes through
It is a meso compound, so it is optically inactive.
cis-1,4-Dichlorocyclohexane (D)
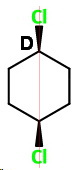 cis-1,4
cis-1,4
This has no chiral carbons, so it achiral and optically inactive.
trans-1,2-dichlorocyclohexane (E and F)
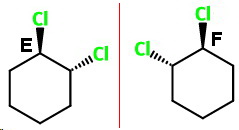 trans-1,2
trans-1,2
This has two chiral carbons, and it has no plane of symmetry. It exists as a pair of optically active nonsuperimposable mirror-image isomers.
trans-1,3-Dichlorocyclohexane
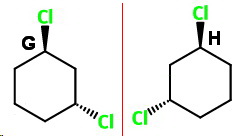 trans-1,3
trans-1,3
trans-1,3-Dichlorocyclohexane has two chiral carbon atoms, and it lacks an internal plane of symmetry.
It is not superimposable on its mirror image. It exists as a pair of optically active nonsuperimposable mirror-image isomers.
trans-1,4-Dichlorocyclohexane (I)
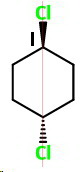 trans-1,4
trans-1,4
This has no chiral carbons, so it is optically inactive.
The enantiomeric pairs are: (E,F) and (G,H).
The diastereomeric pairs are (B,C), (B,E), (B,F), (B,G), (B,H), (C,E), (C,F), (C,G), (G,H), (E,G), (E,H), (F,G), and (F,H).
Meso compounds
The meso compounds are B and C.

