How do I determine the valency of a main group element?
1 Answer
Usually by looking at how many columns away from the noble gases the element is, on the periodic table.
The valency is basically how many bonds it can make, which you can find by drawing the Lewis dot structure and finding which electrons are unpaired at the moment.
Take oxygen (
Drawing in the valence electrons one by one, the first four can be drawn like this:
#cdot stackrel(.)("O")cdot#
#color(white)(.')""^(.)#
Drawing the remaining two, it doesn't really matter where you place them; you'll find that there are two unpaired electrons when you're done:
#cdot stackrel(..)("O"):#
#color(white)(a.)""^(.)#
Or, maybe you could have:
#cdot stackrel(..)("O")cdot#
#color(white)('')""^(..)#
Either way, the Lewis structure tells us that the valency of oxygen atom is
Again, these covalent bonds are made by pairing up each electron with another electron from the other atom, such as carbon or hydrogen.
Two examples are water:
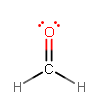
or even something like formaldehyde:
in which it also makes
As a result, you typically see oxygen making two bonds; otherwise, it ends to have a charge, like in