Question #10a9f
1 Answer
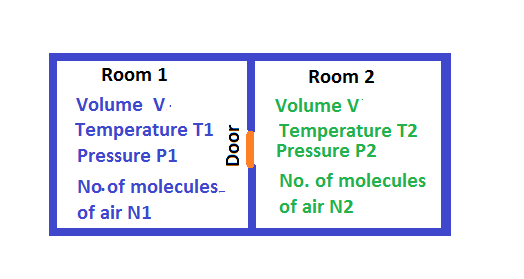 Drawn
Drawn
Temperature, pressure, volume , and number of molecules of airin two identical room are shown in figure.
By equation of state of ideal gas we know that
Where
In our case the rooms are identical . This means they have equal volume .The temperatures of the rooms are different and they are perfectly insulated. So the temperatures are remaining unaltered with time.
If the air in the rooms are in in equilibrium of pressure then
As number of molecules is proportional to number of moles
If
So the room having higher temperature will have less molecules to maintain equilibrium of pressure.

