Question #4034c
1 Answer
Dec 31, 2016
The answer is d.
Explanation:
For an atom to violate the octet rule, it must use its
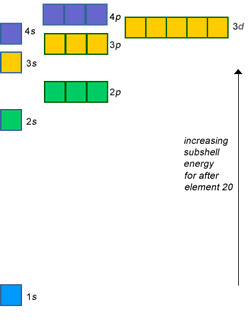
For second row elements like
The energy required to use a
The answer is d.
For an atom to violate the octet rule, it must use its

For second row elements like
The energy required to use a