Question #8928d
1 Answer
Dec 31, 2016
Here's how I would do it.
Explanation:
Ammonium sulfate,
It consists of two ammonium ions,
We must first draw the Lewis structures of each ion. They are:
 ligand-expo.rcsb.org
ligand-expo.rcsb.org
and
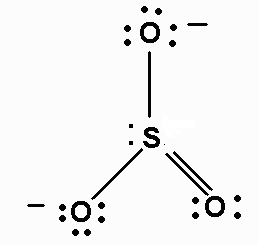 www.chemistry.mcmaster.ca
www.chemistry.mcmaster.ca
Thus, the skeleton structure of ammonium sulfite would be
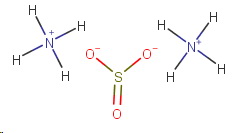 Ammonium sulfite
Ammonium sulfite

