Question #2cd42
1 Answer
Here's my understanding.
Explanation:
Coordinated water is water that is a ligand in a metal complex.
The metal atom and the water molecules are held together by coordinate bonds, as in

Hydrogen-bonded water is the water that that is linked to some other ion by hydrogen bonding.
For example, solid
The
The fifth water molecule is hydrogen bonded to the sulfate ion.
That means the two water molecules on the axial positions of
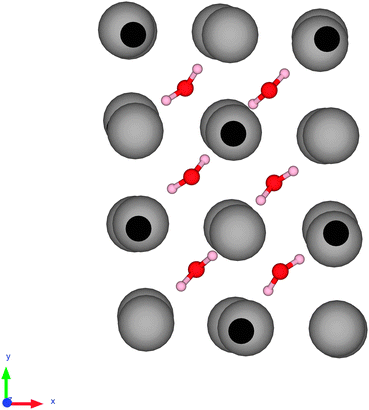
Interstitial water is water that is present in the interstitial sites of a crystal lattice, as in hydrated
In the diagram below, the small dark spheres are