Question #8ac94
1 Answer
The IUPAC name of
Explanation:
There are several possible structures that correspond to your formula.
I will pick one of them.
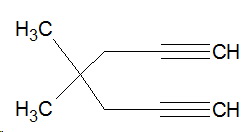
Let's re-draw the structure so it looks like this:
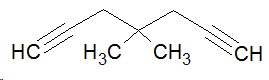
The longest continuous chain now runs horizontally.
To name the compound, you follow these steps.
1. Count the number of carbon atoms in the longest continuous chain.
There are seven carbons, so the name will contain the multiplying prefix hepta.
2. Identify any the multiple bonds in the chain.
There are two alkyne groups, so the ending of the name is diyne (di for "two" and yne for the triple bond).
So far, the name is heptadiyne.
3. Locate these groups on the main chain.
Number the carbon atoms in the chain from 1 to 7.
The triple bonds are between
Insert the lower number of each bond immediately before the "diyne" portion of the name.
Separate the numbers by commas, and use hyphens to separate numbers from letters.
The name is now hepta-1,6-diyne.
4. Identify any other groups on the main chain.
There are two methyl groups, so the name becomes dimethylhepta-1,6-diyne.
5. Assign locating numbers to these groups.
They are both on carbon 4, so the complete name is 4,4-dimethylhepta-1,4-diyne.

