How can I draw trans-3-decene according to IUPAC?
1 Answer
When trying to draw compounds according to IUPAC nomenclature, you have to break down the name into its individual components. The six factors that determine the name of a compound are
- Functional group
- Unsaturation;
- Parent chain;
- Substituents;
- Stereoisomerism;
- Numbering.
Now, let's break down the name trans-3-decene. Since the name ends is
The
The parent chain is designated by
Now for the first part of the name, trans-3. The 3 is used to designate the first carbon of the double bond. This implies that the double bond is located on carbons 3 and 4.
Trans is used to describe the fact that the double bond has two identical groups on opposite sides. In this case, the two hydrogen atoms bonded to carbons 3 and 4 will have a trans relationship.
Now to put all these together again. Start by drawing the parent chain, decane
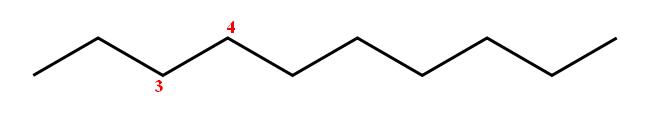
Place the double bond between carbons 3 and 4, like this
Now check for the trans relationship between the two hydrogen atoms attached to the double bond carbons
As you can see, the trans relationship is indeed in place, which means that the bond line notation for trans-3-decene is


