Which of the following statements is correct?
a. The greatest probability of finding a #"1s"# electron is at the nucleus.
b. The size of an orbital is directly proportional to its principal quantum number,
c. A #"3p"# orbital can hold three electrons.
d. A #"2s"# orbital is different from a #"1s"# orbital because the #"2s"# orbital requires more energy for the electrons to fill it.
a. The greatest probability of finding a
b. The size of an orbital is directly proportional to its principal quantum number,
c. A
d. A
1 Answer
d.
Explanation:
a. This option is wrong.
The probability of finding electrons of any orbital in the nucleus is zero.
Here are the probability plots for
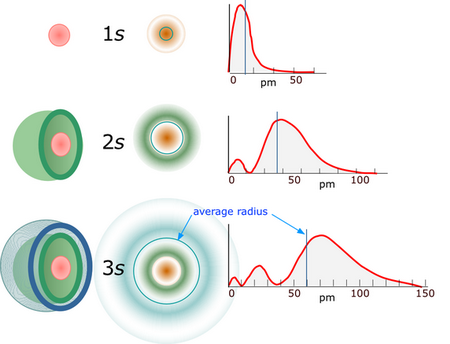
(From Quora)
Notice that the probability is zero at the nucleus (0 pm).
b. This option is wrong.
The sizes of the orbitals are roughly proportional to

Thus, the
c. This option is wrong.
The Pauli Exclusion Principle states that no orbital can hold more than two electrons.
d. This option is correct.
A
Thus, a

