Question #6950a
1 Answer
Here's what I got.
Explanation:
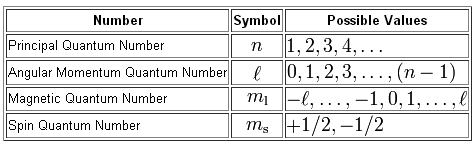
As you know, a full set of quantum numbers contains
Your starting point here will be the electron configuration of a neutral silicon atom, which looks like this
#"Si: " 1s^2 2s^2 2p^6 color(blue)(3)s^2 color(blue)(3)p^2#
Now, by
the last electron of a silicon atom
I assume that you mean the electron that would be added last to the electron configuration of silicon.
The Aufbau Principle states that electrons fill empty orbitals in order of increasing energy levels. In your case, the highest energy level that holds electrons in a silicon atom will be the third energy level.
This means that the principal quantum number,
#ul(n = color(blue)(3))#
The angular momentum quantum number,
- the
#color(blue)(3)s# subshell#-> l = 0# - the
#color(blue)(3)p# subshell#-> l = 1#
The
This means that you have
#ul(l = 1)#
The magnetic quantum number,
#l = 1 implies m_l = {-1, 0 ,1}#
By convention, these three values of the magnetic quantum number designate the following orbitals
#m_l = -1 -># the#p_x# orbital#m_l = color(white)(-)0 -># the#p_z# orbital#m_l = color(white)(-)1 -># the#p_y# orbital
Now, it's important to keep in mind that the two electrons will be added to separate
In other words, and once again by convention, you can say that one of the two electrons will occupy the
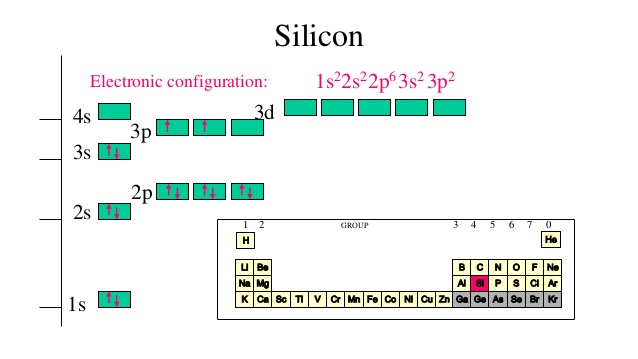
Therefore, these two electrons will have
#m_l = -1" "# and#" "m_l = 0#
Finally, the spin quantum number,
By convention, electrons that are added to an empty orbital are assigned a positive spin, or
#m_s = +1/2#
Therefore, you can say that the two electrons added last to the electron configuration of a neutral silicon atom will have the following sets of quantum numbers
#n = 3, l=1, m_l = -1, m_s = +1/2# This electrons is located on the third energy level, in the 3p subshell, in the
#3p_x# orbital, and has spin-up
#n = 3, l=1, m_l = 0, m_s = +1/2# This electrons is located on the third energy level, in the 3p subshell, in the
#3p_z# orbital, and has spin-up
If you want to pick one electron as the one added last, go with the electron added to the

