Can you account for the stereochemistry of #"1,2-dimethylcyclohexane"#?
1 Answer
Mar 18, 2017
Well, as far as I know, ONLY
Explanation:
Both molecules can support optical isomerism; but ONLY
For
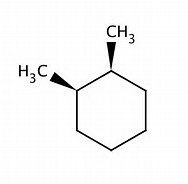
And this cis isomer, clearly has a plane of symmetry bisecting the
On the other hand, for
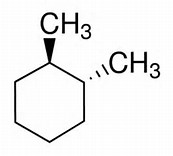
For

