Question #4ee02
1 Answer
It can be solved like this:
Explanation:
1.
This is not a well worded question since the rate of the reaction is not constant. I will assume they are asking for the average rate after 40 min.
Average rate = loss of sulfur / time
Average rate =
2.
There are several methods you could use to show this. I will use "The Integral Method".
For a 1st order reaction we have:
Where k is the rate constant.
This can be expressed in terms of the rate of disappearance of A :
Rearranging and applying integration between 0 and t gives:
This gives:
This means that if the reaction is 1st order then a plot of
The gradient of the line will be equal to -k.
Excel is a useful tool to do this (other spreadsheet programmes are available) . Here's what I got:
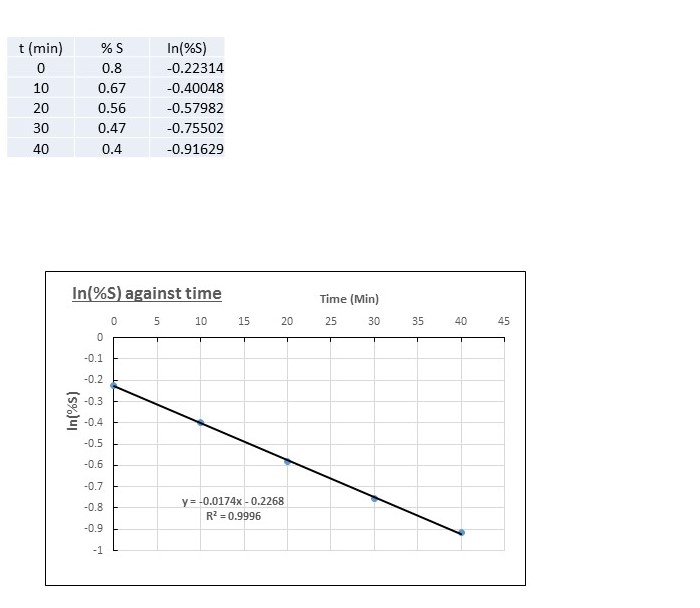
The straight line confirms that the reaction is 1st order.
Since
The equation of the line has been added by the programme for you:
This tells us that
3.
We now have the rate equation for the reaction:
Rate =
At the 30th minute the %S = 0.47

