Question #f1c28
1 Answer
Apr 13, 2017
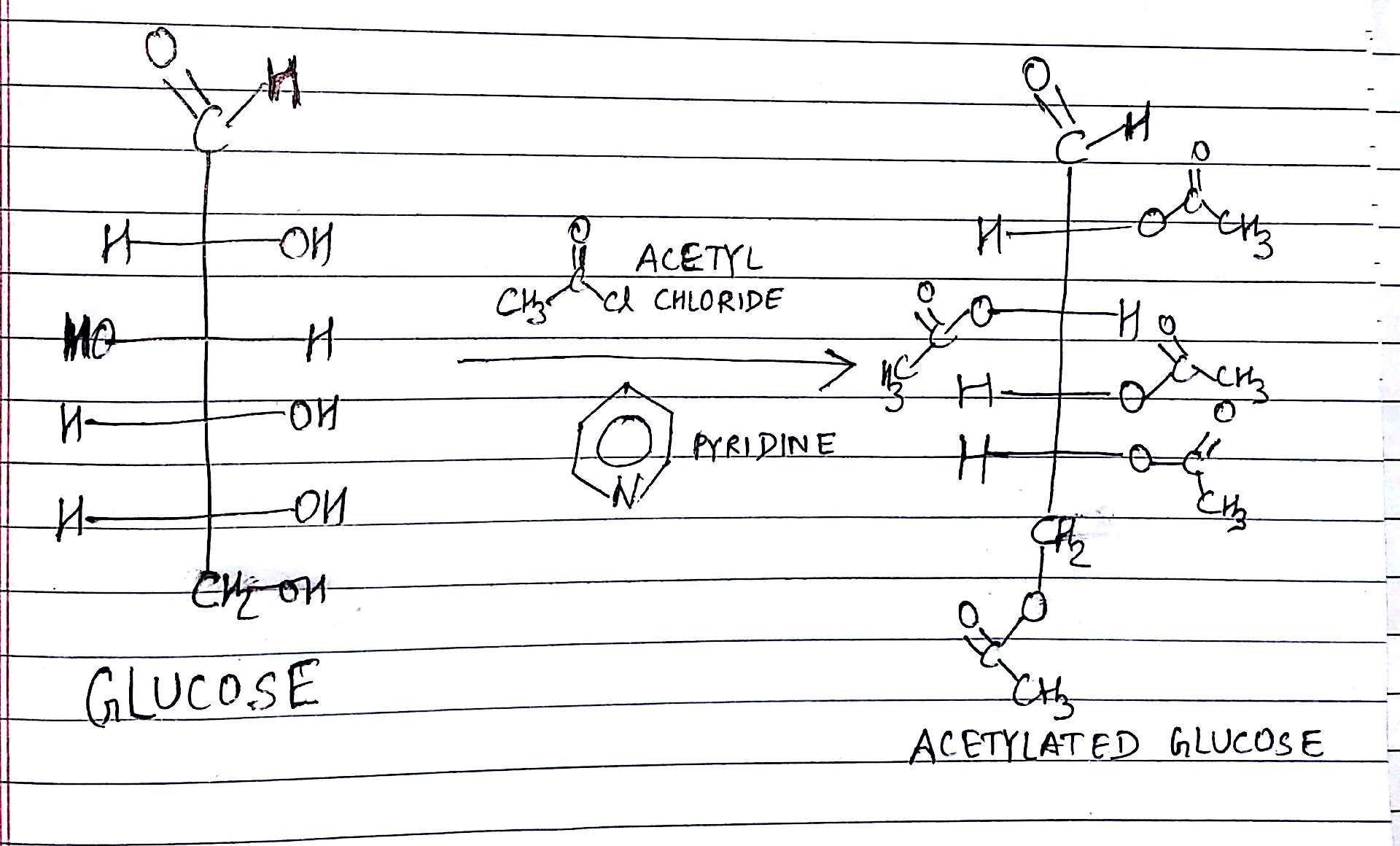
By Acetylation of Glucose. It is explained below:-
Explanation:
Let us say we have 1 mole of Glucose having weight 180 gram.
After Acetylation , the weight of glucose increases by 210 gram because of attachment of 5 Acetyl groups ; each having weight of 43 gram and 5 Hydrogen atoms are also removed from the 5 -OH groups of Glucose.
The net weight gain = (43 x 5) - (1 x 5) = 210 gram
The removal of these 5 Hydrogen atoms Or the attachment of 5 Acetyl groups confirm the presence of 5 - OH groups in it.
The reaction is given as shown below:-