How do we conceive of the covalent bond in terms of the energy of interaction between two atoms?
1 Answer
Traditionally, we regard the covalent bond as the sharing of two electrons between two positively charged nuclei.
Explanation:
Covalent bonding is traditionally regarded as the sharing of electrons between the bound atoms, while ionic bonding is regarded as the transfer of electrons between atoms to form positive and negative particles, called
The modern covalent bond is regarded as a REGION OF HIGH ELECTRON DENSITY composed of 2 electrons that is LOCATED BETWEEN TWO POSITIVELY CHARGED ATOMIC NUCLEI. Such an interaction NEGATES the INTERNUCLEAR REPULSION BETWEEN 2 NUCLEI, so that the nuclei can approach each other closely, and a net attractive force results.
The distance between the 2 nuclei that MAXIMIZES nuclear/electronic attraction, yet MINIMIZES internuclear REPULSION is the equilibrium covalent bond length. And such an interaction can be represented by a graph of potential energy, versus internuclear distance:
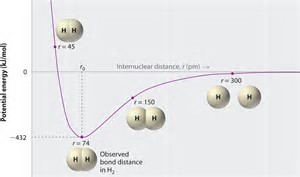
At a LARGE distance of separation, the individual nuclei have little to NO energy of interaction. At small distances of separation,

