For the reaction #2A + B -> 3C#, if the rate of disappearance of #B# is #"0.30 mol/L"cdot"s"#, how do you set up a calculation to determine the rate of disappearance of #A# and appearance of #C#?
1 Answer
For
#2A + B -> 3C# ,
knowing that the rate of disappearance of B is
#(Delta[B])/(Deltat) = -"0.30 M/s"# ,
we just have to check the stoichiometry of the problem. Note that the overall rate of reaction is therefore
Since twice as much
Therefore:
#-1/2(Delta[A])/(Deltat) = -(Delta[B])/(Deltat)#
Similarly, since three equivalents of
#color(blue)(-1/2(Delta[A])/(Deltat) = -(Delta[B])/(Deltat) = 1/3(Delta[C])/(Deltat))#
Knowing that, you can calculate the rate of disappearance of
Even reactions with large negative
The activation energy is high for such reactions, and it is difficult for the molecules to find the opportunity to overcome it. They are simply colliding until a large enough fraction of the molecules have sufficient energy to overcome the reaction barrier.
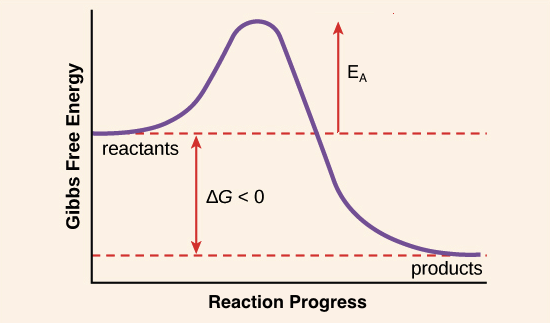
Until enough of them do, the reaction does not occur.

