Explain why photochemical reactions are generally zero order?
1 Answer
A normal, collision-based reaction can only occur if collisions occur frequently enough and with enough energy to overcome an activation barrier.
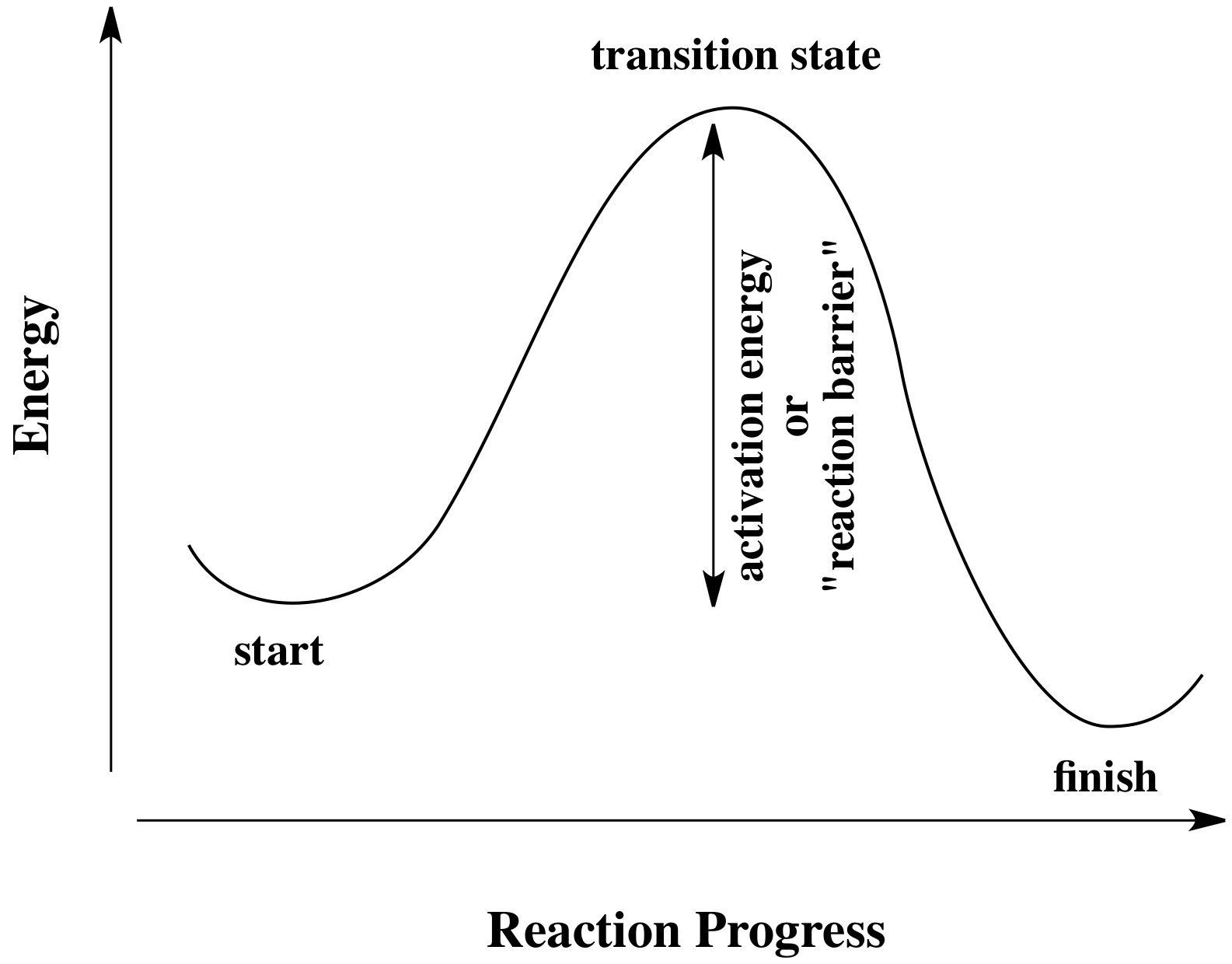
A photochemical reaction, which is NOT collision-based, is a light-induced reaction in which every molecule that the light is shined upon undergoes the reaction by absorbing light of a certain wavelength.
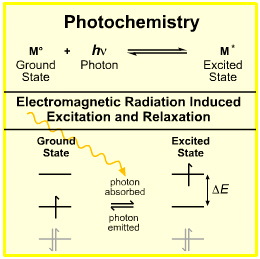
Since energy levels are quantized, only exactly the right wavelength will cause the photochemical reaction to occur. Therefore, it is only a question of whether the molecules undergo the reaction or not, not how close or not close they are to doing so.
As a result, it also does not matter what concentration your molecules are, because again, photochemical reactions involve either overcoming the activation barrier or not, and nothing in between.
And in fact, if concentration does not matter, then the rate law must have a zero order dependence on concentration.
#=> r(t) = k[A]^(0) = k#

