Question #5e4ac
1 Answer
May 2, 2017
First Order
Explanation:
The best way to solve kinetics problems relating rate and time is to use the integrated rate law.
To find the order of the reaction, take the following data points, and graph the following:
- Concentration vs. Time
- Natural Log (ln) of concentration vs. Time
#1/(concentration)# vs. Time
Notice which one best resembles a straight line. Based on which line is straight, you can figure out your order. The following graphic sums it up nicely:
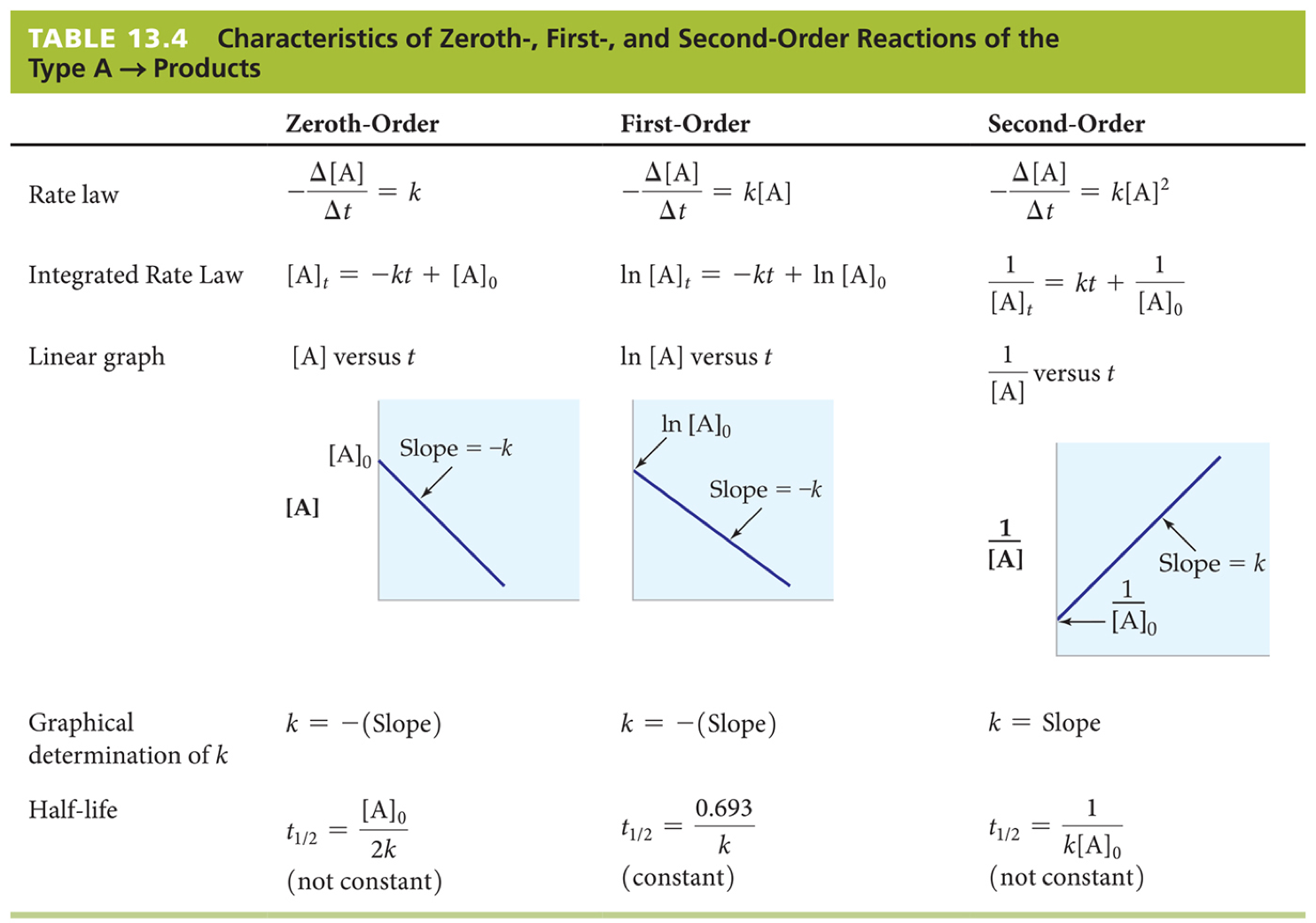
If you graph all of those, you should find that it's a first order reaction.
You can then easily use this information, along with the information in the graphic above, to solve the remaining parts of your problem.
Hope that helps :)

