Explain with a well-labelled diagram, the concept of activation energy, and how a catalyst affects such energy?
1 Answer
Sep 14, 2017
Well, where is your well-labelled diagram?
Explanation:
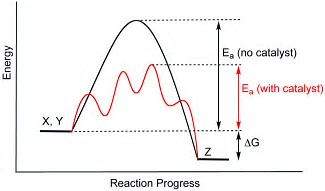
The decisive factor is
If the catalyst LOWERS

