How many spherical nodes are in a 3d_(xy) orbital?
1 Answer
May 20, 2017
A spherical node is otherwise known as a radial node. Radial nodes are given by
n - l - 1 ,where
n - 1 is the total number of nodes, andl is the number of angular nodes. (Ordinarily,n is the principal quantum number, andl is the angular momentum quantum number.)
So, the number of radial nodes is:
color(blue)(n - l - 1)
= 3 - 2 - 1 = color(blue)(0)
Hence, there are zero spherical nodes in the
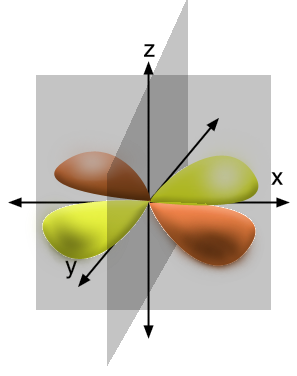
How many angular nodes are there?

