Why isn't allene planar?
1 Answer
May 30, 2017
I assume you mean the twisted propadiene.
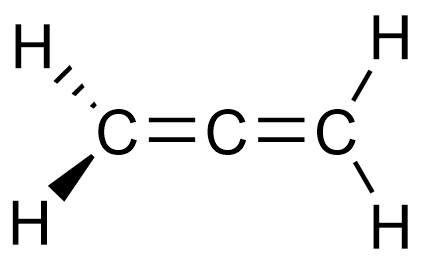
Well, the left half of allene is perpendicular to the right half. In order for a molecule to be planar, the whole molecule has to be planar.
A simplified explanation is that the central carbon atom's
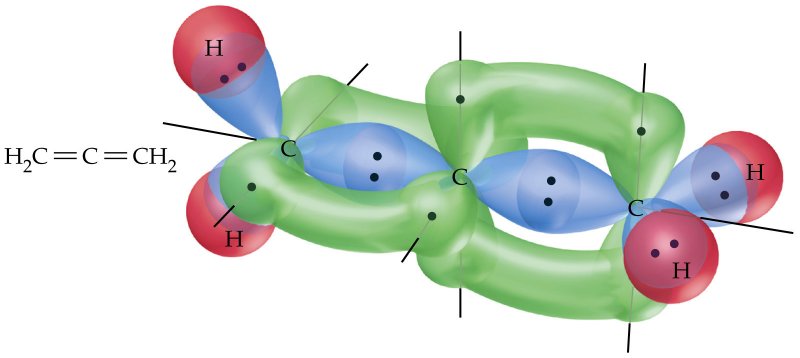
(A planar allene exists, but for a much shorter amount of time than the twisted allene as it equilibrates into the twisted form.)

