Which lanthanides are exceptions to the Aufbau principle?
1 Answer
See here for a fuller explanation on the lanthanides and actinides.
The order by atomic number is down the first column, and then down the second column. In
#color(white)([(color(red)(La),(color(red)([Xe] 6s^2 5d^1)),color(black)(Tb),(color(black)([Xe] 6s^2 4f^9))),(color(red)(Ce),(color(red)([Xe] 6s^2 4f^1 5d^1)),color(black)(Dy),(color(black)([Xe] 6s^2 4f^10))),(color(black)(Pr),(color(black)([Xe] 6s^2 4f^3)),color(black)(Ho),(color(black)([Xe] 6s^2 4f^11))),(color(black)(Nd),(color(black)([Xe] 6s^2 4f^4)),color(black)(Er),(color(black)([Xe] 6s^2 4f^12))),(color(black)(Pm),(color(black)([Xe] 6s^2 4f^5)),color(black)(Tm),(color(black)([Xe] 6s^2 4f^13))),(color(black)(Sm),(color(black)([Xe] 6s^2 4f^6)),color(black)(Yb),(color(black)([Xe] 6s^2 4f^14))),(color(black)(Eu),(color(black)([Xe] 6s^2 4f^7)),color(black)(Lu),(color(black)([Xe] 6s^2 4f^14 5d^1))),(color(red)(Gd),(color(red)([Xe] 6s^2 4f^7 5d^1)),"","")])#
Generally the electron configuration exceptions occur
- early in the lanthanide series (lanthanum, cerium)
- after filling the
#4f# orbitals halfway (gadolinium).
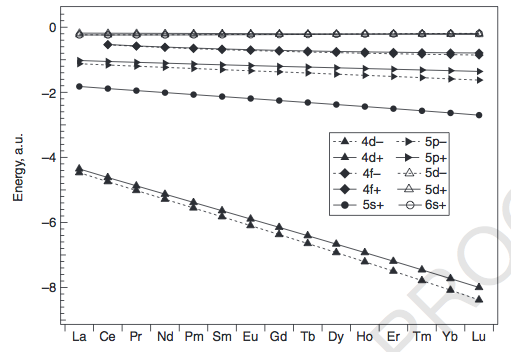
In general, it has to do with the closeness in energy of the
That gives rise to the following rationales:
-
#"La"# and#"Ce"# have an electron in their#5d# instead of#4f# because the#4f# is more radially compact, and given the small difference in energy between them (about#"6.8 eV"# ), it is more energetically favorable to choose the#5d# , which is radially more diffuse. -
#"Gd"# has about a#"16 eV"# #4f-5d# energy gap, and apparently, the electron repulsion it would generate to add another#4f# electron, combined with the radial compactness of the#4f# orbitals, is enough that it would rather have that electron in the#5d# .

