Why is there an exception in the ionization energy trend in the second-row p-block elements?
1 Answer
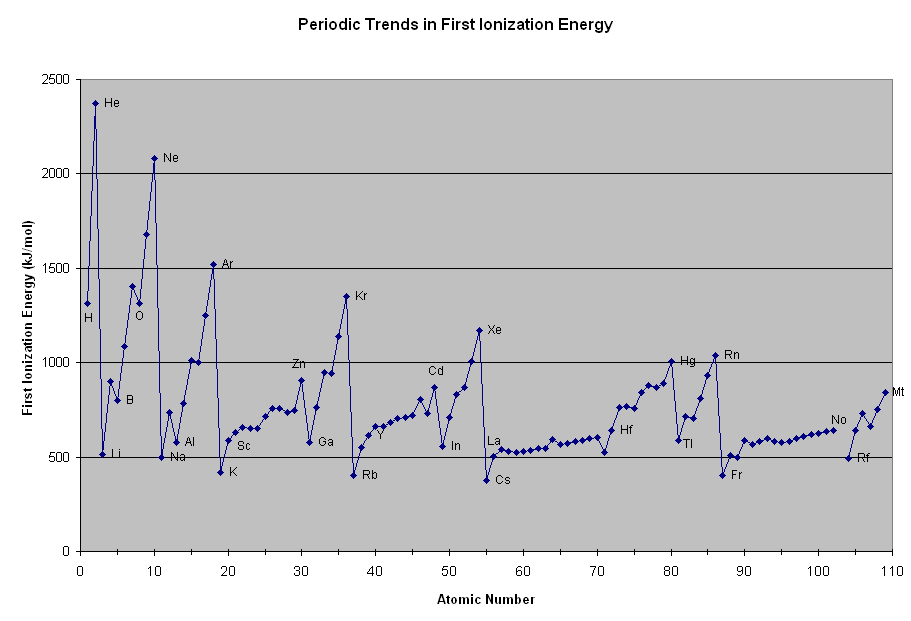
I think you are wondering why nitrogen has an ionization energy that locally peaks in the ionization energy chart:
 https://www.angelo.edu/
https://www.angelo.edu/
(search for the atom one left of
"O" , nitrogen, which is atomic number7 .)
Nitrogen's atomic electron configuration is:
[He]2s^2 2p^3 ,
or more explicitly:
ul(uarr color(white)(darr))" "ul(uarr color(white)(darr))" "ul(uarr color(white)(darr))
underbrace(" "" "" "" "" "" "" "" ")
" "" "" "2p
ul(uarr darr)
2s
All the electrons are unpaired, so that's not the exception here.
The exception arises with
This is due to the atomic electron configuration for
[He]2s^2 2p^4
or more explicitly:
ul(uarr darr)" "ul(uarr color(white)(darr))" "ul(uarr color(white)(darr))
underbrace(" "" "" "" "" "" "" "" ")
" "" "" "2p
ul(uarr darr)
2s
One of the
That is enough to lower the first ionization energy of oxygen atom to be below that of nitrogen atom by about
https://en.wikibooks.org/

