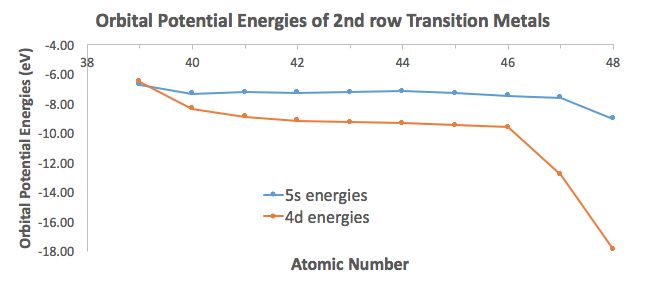
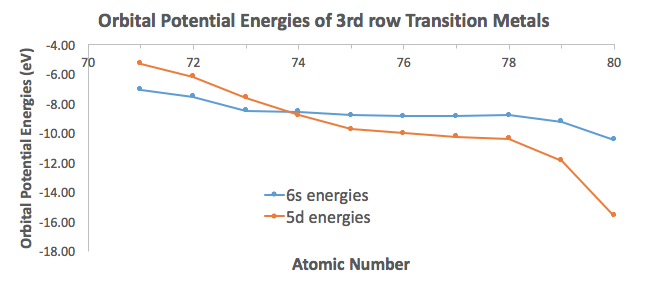
Why does the nsns orbital go before the (n-1)d(n−1)d orbital when writing transition metal electron configurations?
1 Answer
Sep 27, 2017
It doesn't. The
See how the
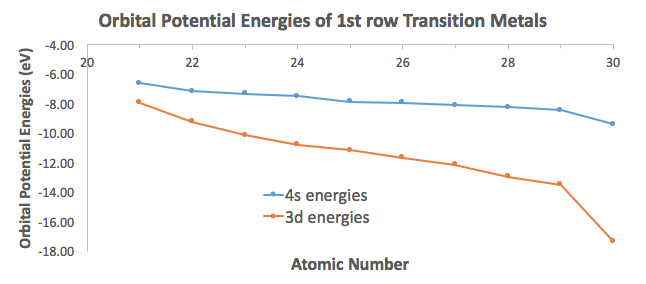
And you can further see how the Aufbau principle fails for the heavier transition metals, in that the