What are the important features of a metal reactivity series?
1 Answer
The important features of a metal activity series are to compare the reactivity of metals and predict whether a single replacement reaction will occur.
Explanation:
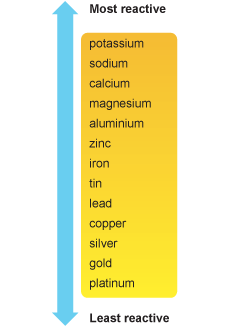
A metal activity series is a list of metals according to their reactivity. The metals at the top of a metal activity series are much more reactive than those at the bottom. In a single replacement reaction, the reactants are a metal and an ionic compound containing another metal. The metal in the reactants can replace the metal in the ionic compound in the reactants, if it is above the other metal in the series.
Example: Will the following single replacement reaction occur?
Answer: If we look at the activity series below, lead
Example: Can the reverse reaction occur?
Answer: Silver is below lead on the activity series. Therefore, the reverse reaction cannot take place.