What net charge will an atom that loses one electron have?
1 Answer
Explanation:
The answer is actually given to you by the question--you know that when potassium becomes an ion, it loses
The resulting ion will thus have
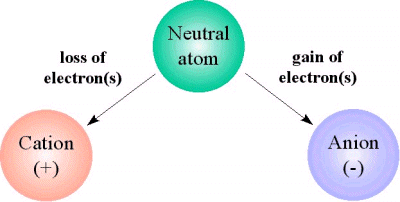
As you know, the net charge of an atom is determined by the ratio that exists between the number of electrons that surround the nucleus and the number of protons located inside the nucleus.
A neutral atom has equal numbers of electrons and protons, and hence a
Now, when an atom loses electrons, the number of protons exceeds the number of electrons
When an atom gains electrons, the number of electrons exceeds the number of protons