Why are there no electronic transitions in #"He"^(2+)#?
1 Answer
Jul 9, 2017
Well, because it has no electrons... And hence there can be no electronic transitions to speak of. But
The Bohr model of course cannot apply... its very definition models the atom as a positively-charged nucleus surrounded by electrons that travel in circular, fixed orbits (that violate the Heisenberg Uncertainty Principle).
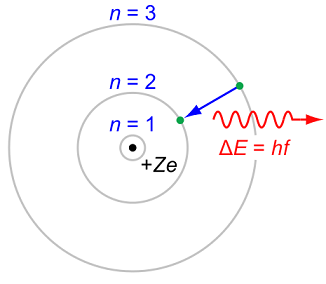
At least hydrogen atom has one electron. But
#"He": 1s^2#
#=> "He"^(2+): 1s^0#
Why does the Bohr model apply to

