Question #a14c2
1 Answer
Jul 20, 2017
Explanation:
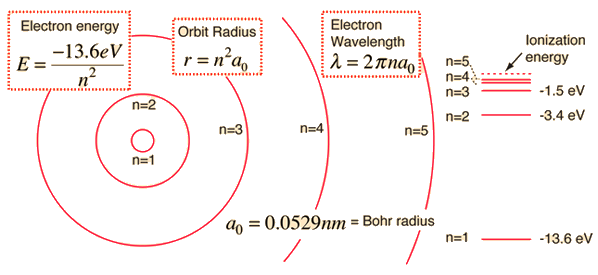
All you need to know in order to answer this question is that the radius of an orbit depends on the principal quantum number,
If you take
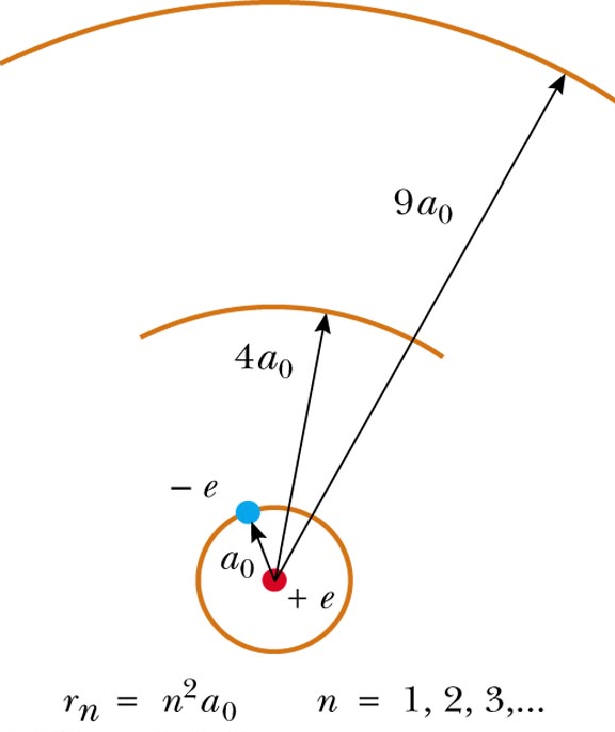
#r_n = n^2 * a_0#
In your case, the third orbit is characterized by
#n = 3#
so you will have
#r_3 = 3^2 * a_0 = 9 * a_0#