Does a catalyst change the thermodynamics of a chemical reaction?
1 Answer
Sep 3, 2017
No...........
Explanation:
Compare the reaction profile of reactions with high and large activation energies.....
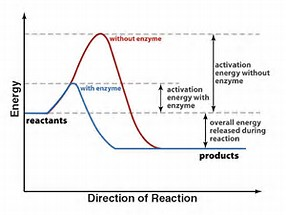
The catalyzed reaction is FASTER than the uncatalyzed reaction given that a greater proportion of the reactant molecules have the requisite activation energy. Of course, we speak of the KINETIC profile of the reaction. The thermodynamics of the reaction are necessarily unchanged and constant. But the higher the

