Question #c7810
1 Answer
Explanation:
Step 1. Draw a skeleton Lewis structure I which every atom has an octet.
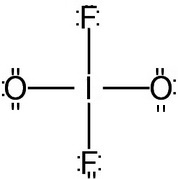
Step 3. Count electrons
The skeleton structure has 32 electrons.
We have available
There is an extra pair of electrons.
They go on the central
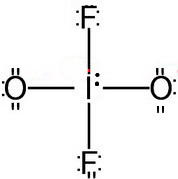
Step 4. Add formal charges
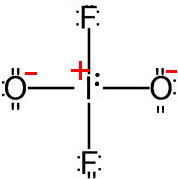
Step 4. Minimize formal charges
Put a double bond to one of the
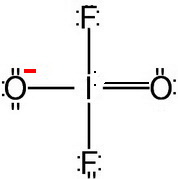
Step 5. Determine the electron geometry
There are four bonded atoms and a lone pair.
This is an
The electron geometry is trigonal bipyramidal.
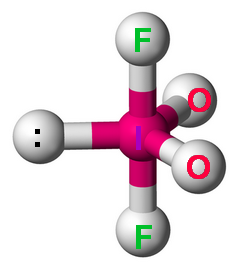
(Adapted from Wikipedia)
The bulky lone pair and the oxygen atoms go in the equatorial positions, and the fluorine atoms go in the axial positions.
Step 6. Determine the molecular geometry
The molecular geometry ignores the lone pair.
The molecular geometry is see-saw.
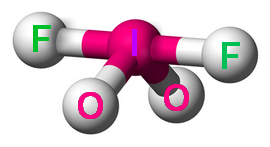
(Adapted from Wikipedia)
So, the structure of
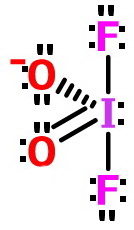
Note: The two
This is just one contributor to a resonance hybrid.

