Design a decay series for a nuclide?
Take the sum of #A=20# , #B=14# , and #C=20# . Add the digits together, then add this result to #90# to get the atomic number of the element you are using. To get the mass number, add #D=22# , #E=02# , and #F=15# , then if it is over #30# subtract #30# until the result is below #30# . If the value is odd and not divisible by #4# , add #1# . Add this result to #230# to get the mass number.
Take the sum of
2 Answers
Well, to find the atomic number, we add
#20 + 14 + 20 = 54#
The sum of these digits is
To find the mass number, we add
#22 + 02 + 15 = 39#
Since the result is over
(If you don't follow, I am literally following the directions on the image.)
So, we currently have
#bb(""_(Z)^(A_m) X = ""_(99)^(240) "Es")# ,
einsteinium-240. I'm guessing that's where you wanted me to stop.
Here's what I get.
Explanation:
Identify the starting nuclide
So, we must devise a decay series for
1. Map a possible decay series
a. Highlight the isotopes that represent the Belt of Stability
Here is a chart I created in Excel.
It shows in yellow the isotopes of the elements from lead to einsteinium, as listed in ptable.org.
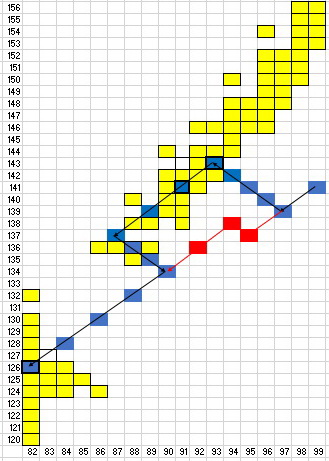
b. Mark the box representing the starting nuclide
c. Mark each isotope in your decay series
The blue boxes represent a decay series that includes as many as possible of the isotopes in the Belt of Stability and ends with
d. Label the type of radiation emitted at each step
The particles emitted are
2. Report the number of
The decay chain involves the emission of eight α particles, four
Note: I much prefer an alternate pathway involving the red blocks in the diagram.
It involves the emission of eight α particles and one


