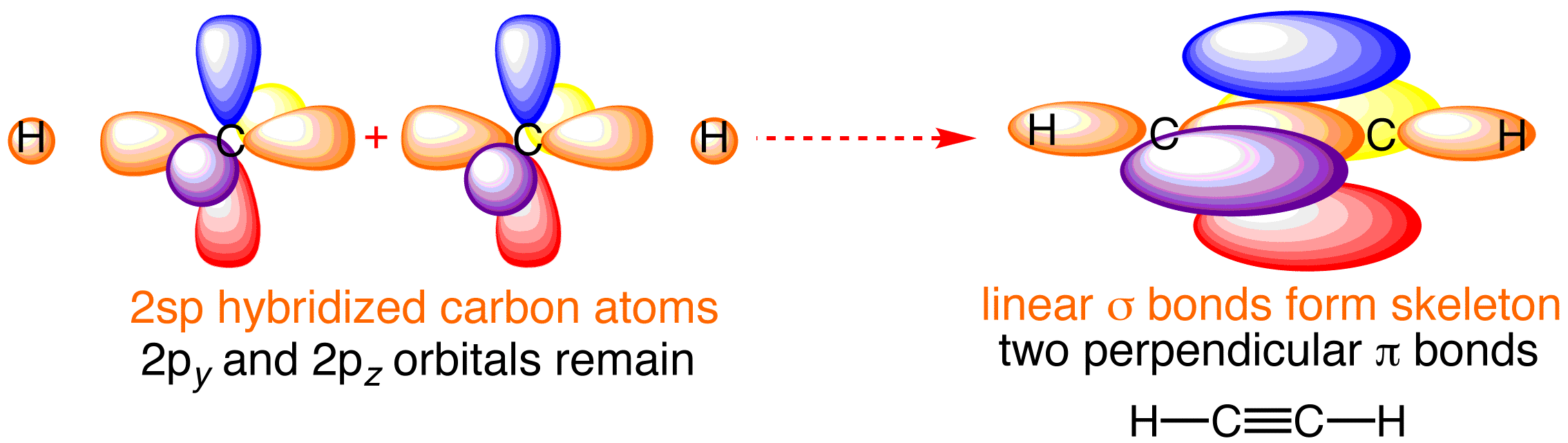
The acetylene molecule features a linear H-C-C-H vector in which participate ALL the valence electrons possessed by hydrogen (1 electron) and carbon (4 electrons) participate. So we have 10 electron to distribute....and these all end up residing in bonding orbitals.
To borrow from valence bond representation, 2 electrons from carbon participate in the linear sigma-C-C and the 2xxC-H bonds; the remaining electrons required for the 2xx terminal sigma-C-H bonds come from the hydrogen, i.e. dotH; i.e. we form 3xxsp hybrid bonds for each carbon centre.
The two electrons remaining on carbon are perceived to be unhybridized, and these are formally p_z and p_y orbitals. These overlap in a pi interaction above and below the plane....
 useruploads.socratic.org)
useruploads.socratic.org)
The electron density BETWEEN the carbon nuclei, negate nucleus, nucleus repulsion, and allows a very short C-C bond length of approx. 1.2xx10^-10*m. This is to be compared to the C-C bond lengths of 1.35xx10^-10*m, and 1.54xx10^-10*m observed for "ethylene" and "ethane", H_2C=CH_2, and H_3C-CH_3 respectively. Dinitrogen, N-=N, another sp interaction also has a short bond of 1.10xx10^-10*m.
 useruploads.socratic.org)
useruploads.socratic.org) 
