How many angular nodes are in a #d_(z^2)# orbital?
1 Answer
Aug 22, 2017
The number of angular nodes in a
Explanation:
For any orbital,
It is easy to see the two angular (conical) nodes in a

A
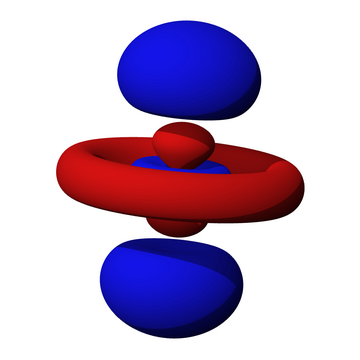
(From Roland Heynkes)
A
(From fineartamerica.com)


