Question #746bf
1 Answer
Sep 1, 2017
Yes, I can think of two methods.
Explanation:
Method 1 — Based on solubility differences
Ammonium chloride is soluble in water, but naphthalene is not.
So, add water to the mixture, stir well, and then filter the mixture.
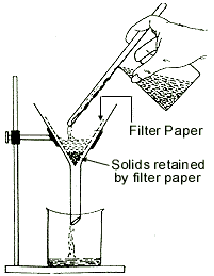
The solid naphthalene will be retained by the filter paper, and you can recover the ammonium chloride by evaporating the water from the filtrate.
Method 2 — Sublimation
Naphthalene undergoes sublimation at approximately 80 °C.
Ammonium chloride also undergoes sublimation, but at a much higher temperature — about 340 °C.
Thus, you can use gentle heating to separate naphthalene from ammonium chloride by sublimation.

The naphthalene will deposit on the cool walls of the inverted funnel, leaving the ammonium chloride in the evaporating dish.

