Question #6b48e
1 Answer
Explanation:
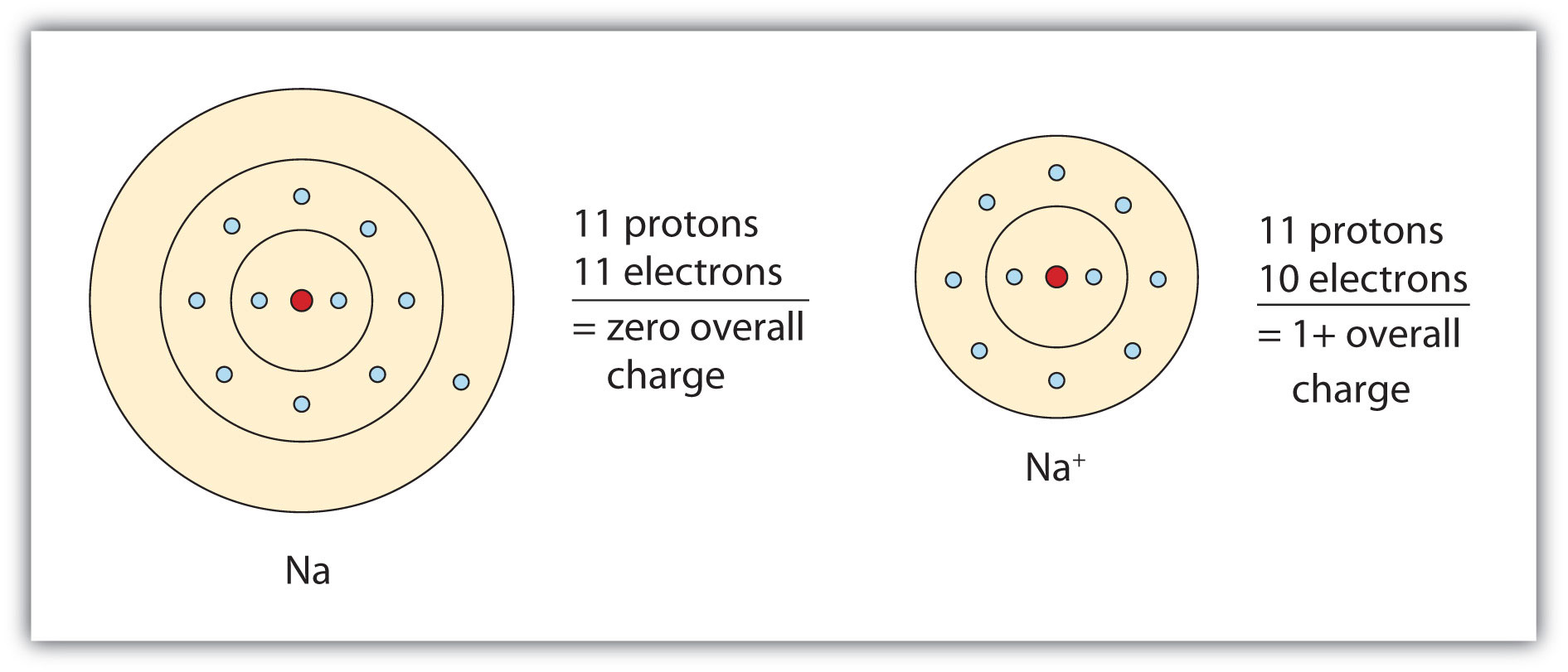
When a neutral atom loses electrons, it becomes a positively charged ion, or cation, because the overall charge of an atom is given by
#color(blue)(ul(color(black)("net charge" = "no. of protons" - "no. of electrons")))#
As long as an atom has equal numbers of protons inside its nucleus and electrons surrounding its nucleus, it will be electrically neutral, .e. it will have a
Now, when an atom loses an electron, the number of electrons that surround the nucleus will decrease by
For example, let's say that a neutral atom starts with
#"net charge" = n - n = 0#
When this atom loses an electron, the number of electrons will go from
This time, the net charge will no longer be equal to
#"net charge" = n - (n-1)#
#"net charge" = color(red)(cancel(color(black)(n))) - color(red)(cancel(color(black)(n))) + 1#
#"net charge" = 1#
At this point, the atom becomes a cation that has an overall charge of
For a numerical example, take an atom of sodium,