Question #9dc3c
1 Answer
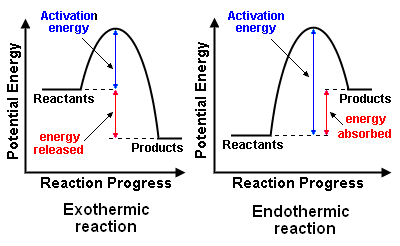
Endothermic.
Explanation:
The idea here is that you need to compare the difference between the energy level of the products and the energy level of the reactants.
You can have
#"energy of products " > " energy of reactants" implies "endothermic reaction"#
or
#"energy of products " < " energy of reactants" implies "exothermic reaction"#
In your case, you have
#overbrace("120 kJ")^(color(blue)("for the products")) " " > " " overbrace("50 kJ")^(color(blue)("for the reactants"))#
you can say that you're dealing with an endothermic reaction.
Another thing to notice here is that the difference between the energy of the activated complex and the energy of the reactants is higher than the difference between the energy of the activated complex and the energy of the products.
#"150 kJ " - " 50 kJ = 70 kJ" -># for the reactants
#"150 kJ " - " 120 kJ = 30 kJ" -> # for the products
This is consistent with the fact that energy is being absorbed by the reaction, i.e. with the fact that you're dealing with an endothermic reaction.
Moreover, you can say that the difference between the energy of the products and the energy of the reactants
#"120 kJ " - " 50 kJ = 70 kJ" -># energy absorbed
constitutes energy that was absorbed by the reaction. Similarly, you can find the activation energy of the reaction by calculating the difference between the energy of the activated complex and the energy level of the reactants.
#"150 kJ " - " 50 kJ = 100 kJ" -># activation energy