Question #89d4c
1 Answer
Explanation:
Start by looking for calcium,
This tells you that an atom of calcium contains
You can thus say that the electron configuration of an atom of calcium must account for a total of
As you know, you can use the Aufbau Principle to write the full electron configuration for an atom of calcium.
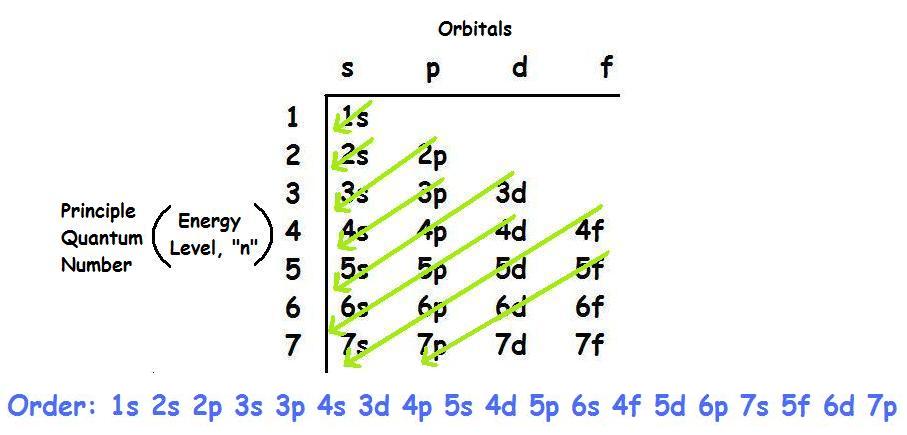
You will end up with
#"Ca: " 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2#
Now, when calcium loses two electrons, it becomes a cation with an overall
#"Ca: " 1s^2 2s^2 2p^6 3s^2 3p^6 color(red)(cancel(color(black)(4s^2)))#
This implies that the full electron configuration of a calcium action will look like this
#"Ca"^(2+): 1s^2 2s^2 2p^6 3s^2 3p^6#
Finally, to write the abbreviated electron configuration of a calcium cation, you need to look in the Periodic Table for the noble gas that comes immediately before calcium.
This noble gas is neon,
#"Ne: " 1s^2 2s^2 2p^6 3s^2 3p^6#
Notice that this is the same electron configuration as the one you have for the calcium cation. To show this, you can write
#"Ca"^(2+): ["Ne"] -># the nogle gas shorthand of a calcium cation
Here
Notice that you can write the noble gas shorthand of an atom of calcium like this
#"Ca: " ["Ne"] 4s^2#
Once again, the

