Point group of #"NF"_3#
#"NF"_3# has a trigonal pyramidal geometry. It belongs to point group #"C"_text(3v)#, like ammonia.
It has a #"C"_3# principal axis and three #σ_text(v)# vertical planes of symmetry.
The image below shows the three #σ_text(v)# planes.
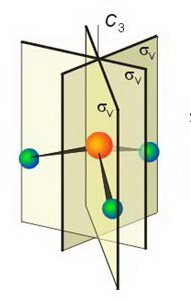
(Adapted from SlidePlayer)
If you look at the molecule from the top, the #"C"_text(3)# axis becomes more apparent. You can rotate #120^@# about the #z# axis to return an indistinguishable configuration of the molecule.
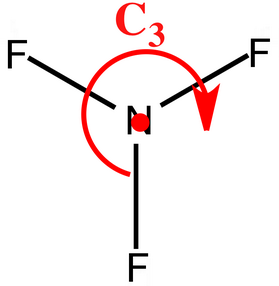
1. #"NF"_3# to #"NClF"_2#
#"NClF"_2# is also a trigonal pyramidal molecule. It belongs to point group #"C"_text(s)#, however, just like if you slightly tilted one #"F"# inwards on #"NF"_3#.
Its structure is
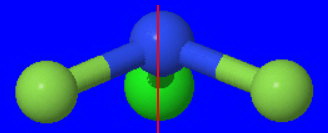
Its only symmetry element is a single #σ_text(h)# plane that bisects the molecule into two mirror image halves.
Thus, it has lost two #sigma_text(v)# mirror planes and its #C_3# axis of rotation.
2. #"NClF"_2# to #"NBrClF"#
#"NBrClF"# is a trigonal pyramidal molecule, but it has no symmetry element except the trivial one of rotating 360° about a #"C"_1# axis.
That now belongs to point group #"C"_1# since it has only the identity element.
Its structure is
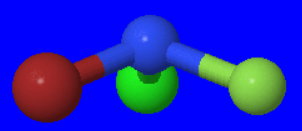
Thus, it has lost even the #sigma_text(h)# plane of #"NClF"_2# .






