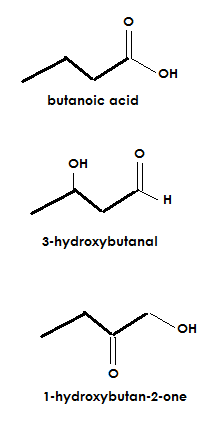
A molecular formula is #C_4H_8O_2#. It has a degree of unsaturation of 1. Also it has an #OH# group, #CH_3# (sp3) group, and #C=O# group. What functional groups can be present?
1 Answer
Carboxylic acid, alcohol, ketone, and aldehyde
Explanation:
A saturated hydrocarbon is one in which there exist no double bonds and no rings within the structure. In other words, if you had a 6-carbon molecule, it would be a chain of 6 carbons and each carbon would be attached to 4 different atoms.
Conversely, an unsaturated compound WILL have a double bond, triple bond, a ring, or any combination of the three depending on the degree of unsaturation.
Degree of unsaturation can be calculated using the following equation:
Where
Based on the formula given in the problem,
This implies that there is one double bond, or one ring present in the molecule. Since
A few possibilities given the conditions of the problem would be: