A reaction is endothermic with H=100 kJ/mol. If the activation enthalpy of the forward reaction is 140 kJ/mol, what is the activation enthalpy of the reverse reaction? I know the answer is 40 kJ/mol but I’m not quite sure how to get there, thanks!
1 Answer
Jan 5, 2018
Explanation:
Here's a schematic progress of reaction diagram for an endothermic reaction.
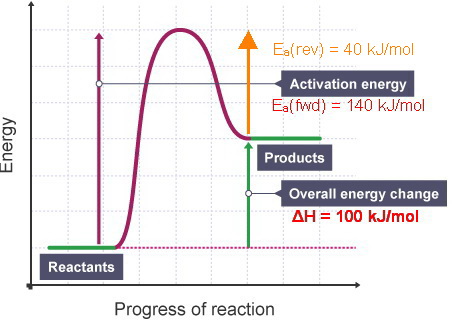
(Adapted from BBC)
We see that
We also see that
Thus,

