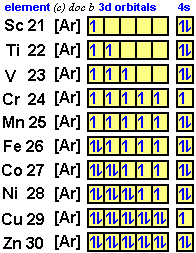
A single orbital in the 3d level can hold how many electrons?
1 Answer
Nov 12, 2016
A single 3d orbital can contain a maximum of two electrons with opposite spins.
Explanation:
Each orbital in any energy sublevel, whether it be an s, p, d, or f sublevel, can contain a maximum of two electrons with opposite spins.