A uranium-235 sample starts with 200 atoms, and 700 million years later, there are 100 atoms. What is the half-life of uranium-235?
1 Answer
Explanation:
The is a great example of a problem that wants to test your understanding of what exactly we mean when we say half-life.
The half-life of a radioactive nuclide,
In other words, if you start with number of atoms and wait until half of them undergo radioactive decay, the period of time needed for this to happen is nuclide's half-life.
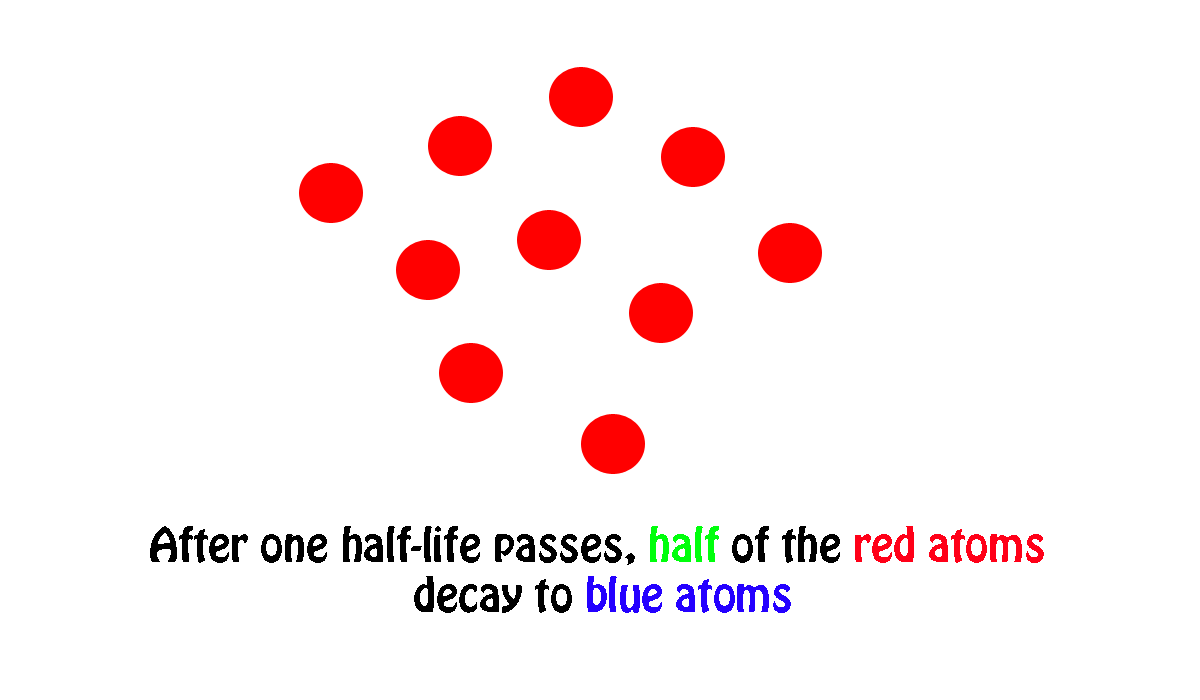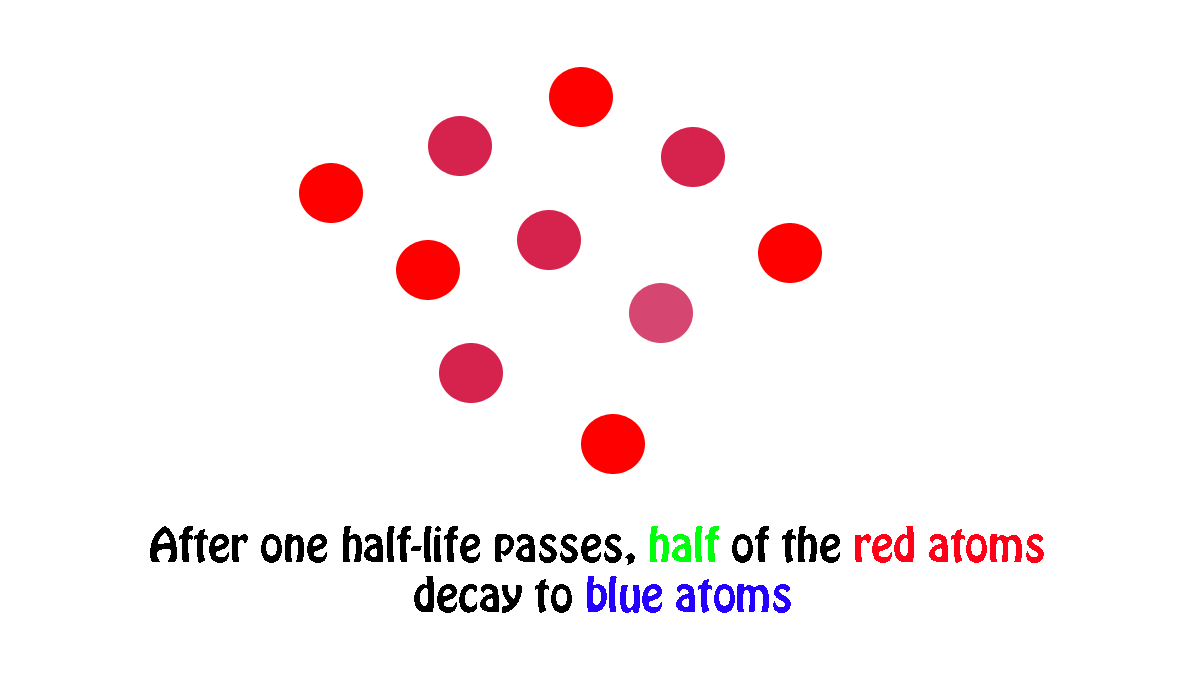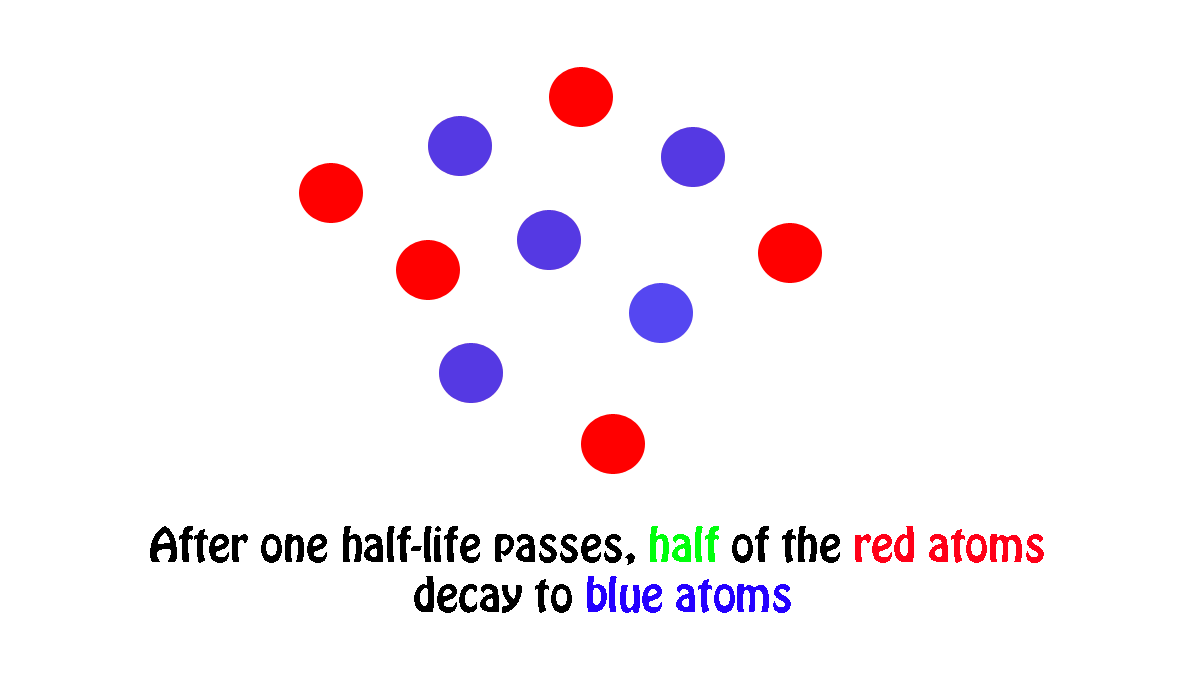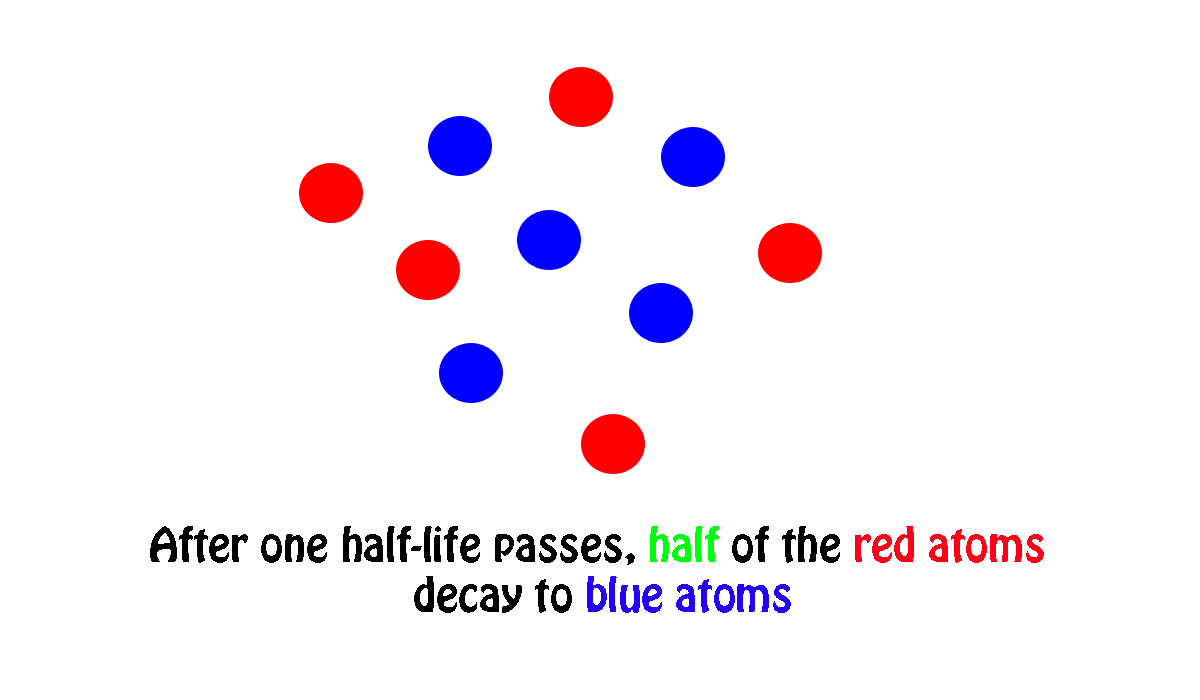 My own work
My own work
In your case, a sample of
Since
"200 atoms" * 1/2 = "100 atoms"200 atoms⋅12=100 atoms
it follows that the time needed for your sample to decay from
Therefore, you can say that
color(green)(|bar(ul(color(white)(a/a)color(black)(t_"1/2" = "700 million years")color(white)(a/a)|)))

