According to the Bohr model of an atom, what happens when an electron moves from the second energy level to the third energy level and then back to the second energy level?
1 Answer
May 4, 2017
The electron absorbs a quantum of energy and moves to the third energy level; going back to the second it releases the energy in form of a photon carrying the energy
Explanation:
Your transition should be located in the so called Balmer Series:
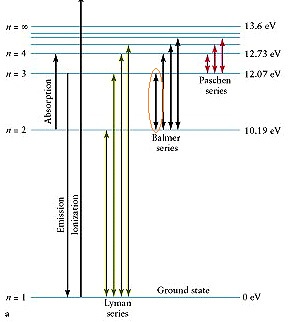
in emission there should be a photon in the visible at

