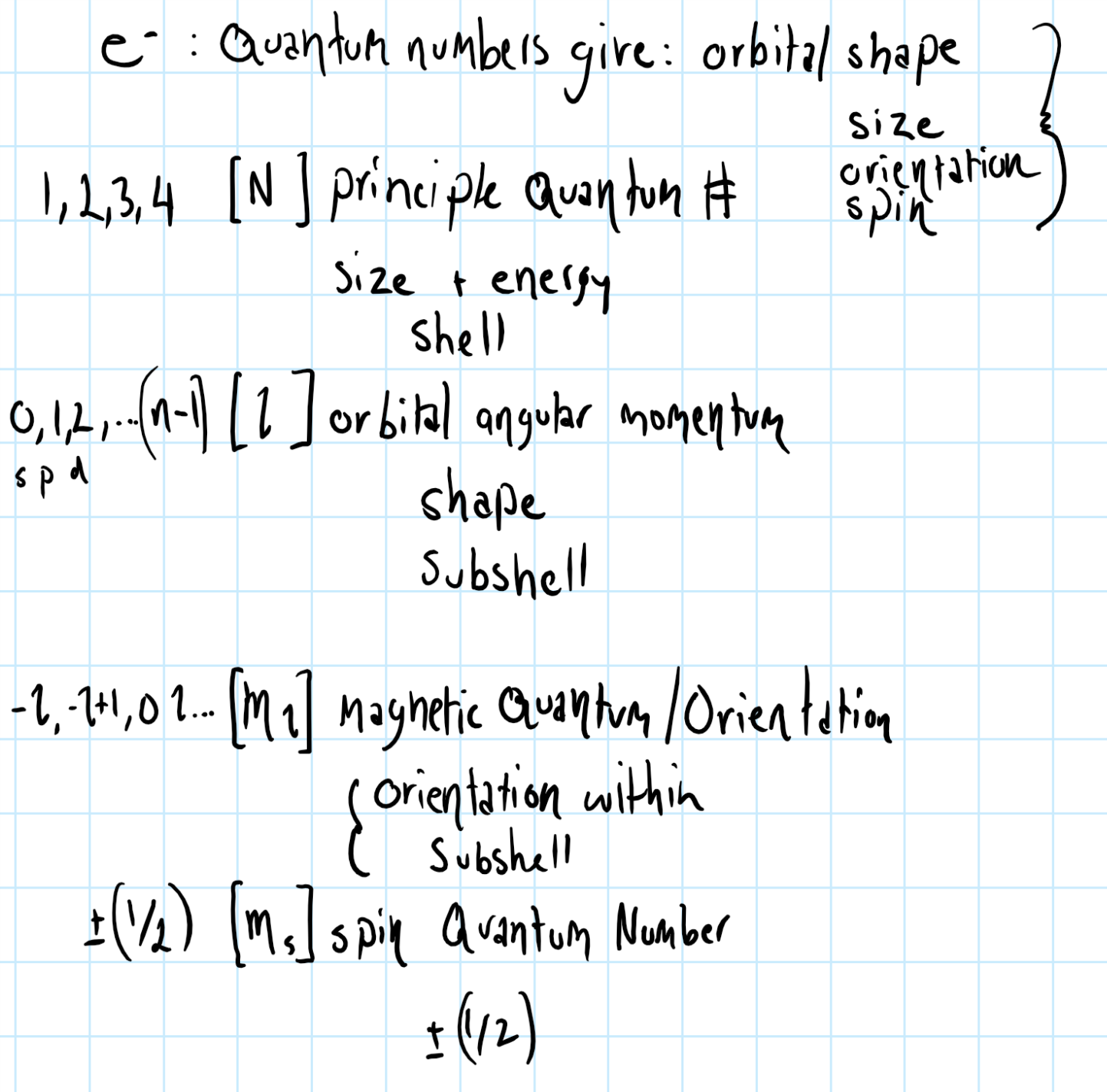An orbital with #l = 0# is what type of orbital? What shape does it have? How many total orbitals does this type have for each principal energy level?
1 Answer
Dec 6, 2016
For
There is one orbital in the
The shape of an s orbital is spherical.
Explanation:
For a higher value of
Consider the graphic below, which shows quantum number notation: