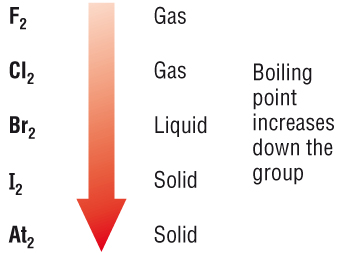
Arrange each group of compounds in order of increasing boiling point. Explain?
#F_2# , #Cl_2# , #Br_2#
1 Answer
Explanation:
As you know, a molecule's boiling point depends on the strength of the intermolecular forces of attraction its molecules exhibit.
In your case, you have to find how the boling points of three nonpolar molecules relate to each other.
The halogens, which are the lements that make up group 17 of the periodic table, exist as diatomic molecules. Since these molecules are nonpolar, they will only exhibit weak London dispersion forces.
Now, London dispersion forces are actually caused by instantaneous and random distorsions in a molecule's electron cloud.
These distorsions take place when instantaneous changes in how the electrons are distributed in the molecule's electron cloud lead to regions that have a higher electron density and regions that have a lower electron density.
In regions with higher electron density, partial negative charges arise. Likewise, regions with lower electron density exhibit partial positive charges.
This leads to the creation of a temporary dipole moment. When a molecule that exhibits such dipole moment comes near another molecule, it polarizes its electron cloud, i.e. it induces a similar distorsion in the second molecule's electron cloud.
This means that for very short period of time, the two molecules are attracted to each other.
As you know, the stronger the intermolecular forces of attraction, the higher th boiling point. This means that the halogen molecule that exhibits the strongest London dispersion forces will have the highest boiling point.
Now, as you go down the group, the size of the atoms, and implicitly the size of their electron clouds, increase. This means that the instantenous distorsions in the electron distribution will create more poweful temporary dipoles.
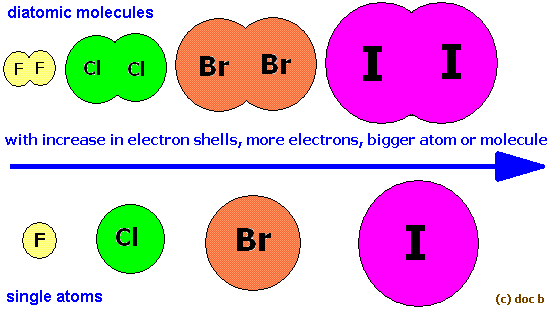
Think of it like this - the bigger the surface are and the magnitude of the partial charges, the stronger the atraction.
Therefore, you can predict that bromine,
By the same logic, fluorine,